periodic table for dummies|periodic table explained for dummies : Bacolod Periodic tables generally display two numbers with each element. The smaller number is the atomic number. This is the number of protons, which is unique to each element and doesn’t change. The .
• Olofsson, Clark (1986). Rättvisans lotteri [The lottery of justice] (in Swedish). Stockholm: Prisma. ISBN 91-518-2030-7. SELIBR 7407420.• Olofsson, Clark (2015). Vafan var det som hände? [What the hell happened?] (in Swedish). Stockholm: Upp med händerna i . Ver mais
0 · understanding periodic table for dummies
1 · periodic table metal vs nonmetal
2 · periodic table for idiots
3 · periodic table for dummies pdf
4 · periodic table for 7th graders
5 · periodic table explained for dummies
6 · periodic table cheat sheet
7 · easy to understand periodic table
8 · More
Resultado da 23 de mai. de 2023 · Deborah Secco - Peça Rara. Deborah Secco é sócia do brechó Peça Rara, que tem mais de 100 unidades espalhadas pelo Brasil — Foto: .
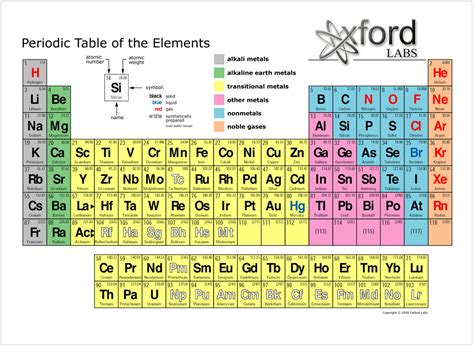
periodic table for dummies*******The periodic table can be used to determine the following properties of materials: Atomic number (Z): Elements are all organized according to their atomic . The periodic table organizes elements into groups and periods based on their chemical and physical properties. Elements in the same group share similar characteristics, like reactivity. The .
Learn about the history, structure and properties of the periodic table with this comprehensive guide for high school students. Find out how to use the table, see photos of each element and explore their uses and applications.
By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. Devised by Russian chemist Dmitri Mendeleev .Chemistry archive. Unit 6: Periodic table. About this unit. This unit is part of the Chemistry library. Browse videos, articles, and exercises by topic. Introduction to the periodic . Periodic tables generally display two numbers with each element. The smaller number is the atomic number. This is the number of protons, which is unique to each element and doesn’t change. The .
periodic table for dummies periodic table explained for dummies Although the Periodic Table of Elements looks complex, it is easier to follow once you learn how to use it. For more free educational resources, visit http:/.The periodic table of elements puts all the known elements into groups with similar properties. This makes it an important tool for chemists, nanotechnologists and other .The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows ("periods") and columns ("groups"). It is an icon of chemistry and is widely used in .periodic table for dummies Using the periodic table, you can classify the elements in many ways.One useful way is by metals, nonmetals, and metalloids. The periodic table is organized in families and periods. Metals In the .periodic table explained for dummiesThe periodic table helps chemists classify elements by properties and similarities. One way to sort the elements is to divide them into three categories: metals, nonmetals and metalloids: Most of the elements on .
A complete guide to the history and organization of the period table. Fill out the worksheet as you watch the video: https://docs.google.com/document/d/1DOPX. The periodic table organizes elements into groups and periods based on their chemical and physical properties. Elements in the same group share similar characteristics, like reactivity. The .
Chemistry: 1001 Practice Problems For Dummies (+ Free Online Practice) In the periodic table of elements, there are seven horizontal rows of elements. Each of these rows are called periods. The vertical columns of elements are called groups, or families. The most common way the periodic table is classified is by metals, nonmetals, and metalloids .By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834–1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element’s .
Inorganic chemistry deals with all the elements of the periodic table. For this reason it’s extremely useful for understanding the world around you — everything from the chemistry of interstellar space, planets, and our own environment here on planet Earth, to the way that Silicon Valley uses silicon to drive the information age forward.
Organic Chemistry I For Dummies. A pattern of repeating order is called periodicity. In the mid-1800s, Dmitri Mendeleev, a Russian chemist, noticed a repeating pattern of chemical properties in elements. Mendeleev arranged the elements in order of increasing atomic mass, to form something that resembles the modern periodic table.
The basic chemistry of acids and bases. A lot of chemistry requires you to understand the difference between acids and bases. An acid is a substance that donates an H + ion to another chemical species called a base. A base is a substance that accepts (combines with) an H + ion.. If you need to know the concentration of the H + ion in .
Hank gives us a tour of the most important table ever, including the life story of the obsessive man who championed it, Dmitri Mendeleev. The periodic table.Atomic radius trends on periodic table. Atomic and ionic radii. Mini-video on ion size. Ionization energy trends. Ionization energy: period trend. First and second ionization energy. Electron affinity: period trend. Electronegativity. Electronegativity and bonding.
In this video, I cover several periodic table basics that will help you read and use the periodic table.The periodic table has a large amount of information .
A hydrogen atom has 1 proton, helium has 2, lithium has 3, and so forth through the periodic table. The atomic number is the number of protons for each element. Except for the simplest hydrogen atom with a single proton as its entire nucleus, all atoms contain neutrons (particles that are electrically neutral) in addition to protons.
Step 8: Increasing Atomic Number. You may have noticed that the periodic table is also arranged in order of increasing atomic number. Hydrogen (H) is the first element with an atomic number of 1. Beginning with hydrogen in the top left of the table, atomic numbers increase from left to right and top to bottom. The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row (called a period) and column (called a group). The .

26. Fe. Iron. You’ll notice that elements in the periodic table are arranged in rows and columns. Rows are called periods. Columns are called groups. Elements in a group (column) have the same number of outer electrons, so they have similar chemical properties. There is an obvious pattern to look for:The periodic table is organized by atomic mass and atomic number. Within the periodic table there are lots of trends as well. These trends are patterns that occur within the periodic table. The three main trends we will study are ionization energy, atomic radii, and electronegativity. However much energy it takes to remove an atom is Ionization .On the periodic table, elements are listed in order of increasing atomic number. Elements in the same row are in the same period. This means they have similar physical properties, such as how well they bend or conduct electricity. Elements in the same column are in the same group. This means they react with other elements in similar ways.
Resultado da Assista os melhores vídeos de Novinhas (18+) no mundo de graça no Pornhub. As melhores pornstars tiram a roupa e fazem sexo explícito nos melhores filmes online de Novinhas (18+). O Pornhub.com tem orgulho de compilar a maior coleção dos mais amados vídeos pornô de Novinhas .
periodic table for dummies|periodic table explained for dummies